Why we take so much care to clean your metal surfaces thoroughly prior to powder coating and adhesive bonding
Making sure that surfaces are clean prior to any adhesive bonding or coating operation is essential. Surface contaminants, many that can’t even be seen, will impair the performance of the adhesive or coating, reducing the bond strength and ultimately leading to premature failure of the system. Thus, the cleaning of the metal involves a lot more than a quick wipe down with a damp cloth.
Powdertech Surface Science have in-house capability, in our dedicated Surface Characterisation Laboratory, to measure the cleanliness of surfaces and to quantify the strength of bonded joints. We use Dyne inks and UV-Fluorescence to measure the level of organic contamination on a surface and show compliance to specifications with RFU limits on in-coming parts. Our pre-treatment procedure for cleaning metal and creating a passivation layer uses chrome-free TiZr systems consisting of six stages with de-ionised water rinses between the stages of acid etch cleaning and conversion coating (passivation).
What are the main contaminants found on metal?
Contaminants will be present, even if the metal looks clean. Table 1 gives a list of some of the more commonly found surface contaminants and their impact on adhesive bonding performance.
Main issues caused by contaminated surfaces
Bond Integrity
Bond strengths will be unacceptably low and this will be even more apparent in wet environments when surface corrosion can initiate. Weld surfaces also require good cleaning to remove volatile contaminants which can lead to popping and spattering during welding and creates porosity within the weld and inconsistent weld quality
System Longevity
More demanding applications require a more thorough clean to ensure the longevity of the system. Battery Electric Vehicles (BEV) are a good example where a high levels of cleanliness in the component structure is vitally important to ensuring the integrity and safety of the battery systems.
Aesthetics
For painting and powder coating a poorly cleaned surface may result in coating defects such as fisheyes, inclusions, pinholes and reticulation. Furthermore, the surface contamination can go unnoticed resulting in field failures due to blistering and corrosion and eventually leading to paint peeling away altogether.
How clean is clean and how do you measure it?
A good question. Here at Powdertech Surface Science we have been researching this topic to find answers for ourselves and our clients. We have made significant investments in equipment and facilities to be able to quantify the cleanliness of a surface prior to adhesive bonding or coating operations.
Figure 2 Liquid droplet wetting a solid surface
Table 1 indicates that contaminants can reduce “the wetting of polymeric based adhesives” but what is exactly is wetting?
Wetting is the process by which an adhesive or coating comes into intimate contact with the substrate surface. Surface energy (or tension) is the key to this process. For good wetting to occur the surface energy of the substrate should be higher than the liquid (the coating or adhesive) to allow a favourable surface interaction and allow the liquid coating to spontaneously spread, see Fig 2.
Fig 3. Typical surface energy of common materials
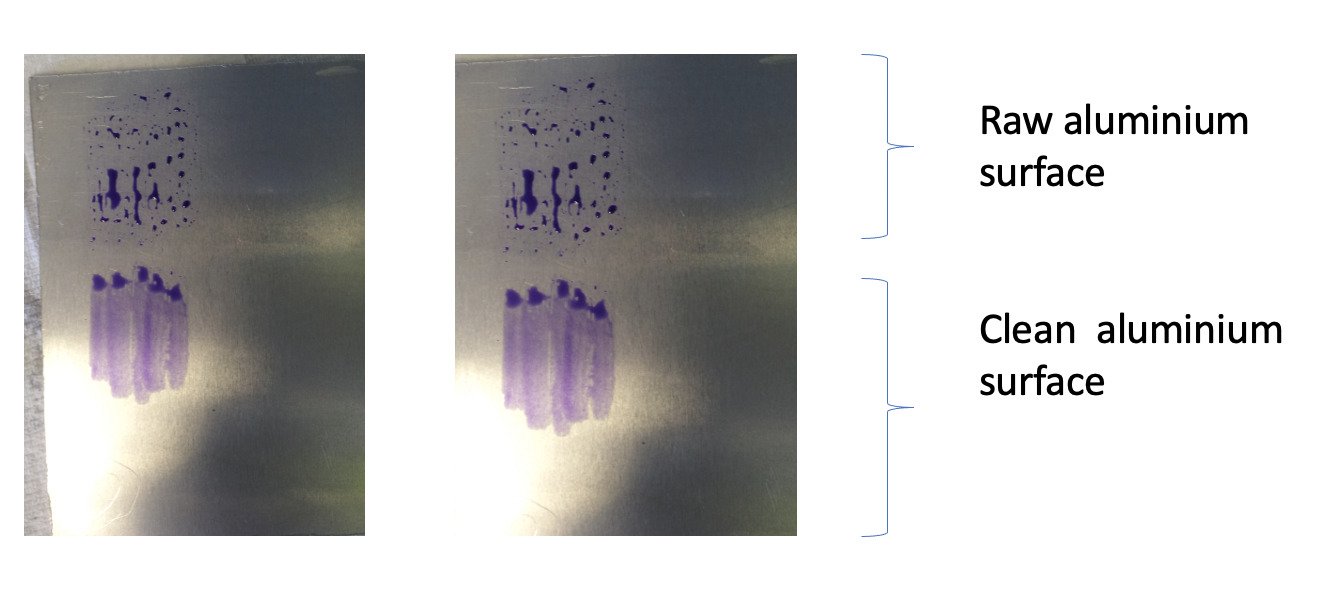
The surface energy of common epoxy-based adhesives is around 45 Dynes/cm so this suggests that the adhesive should spontaneously wet the metallic surface. However, when we actually measure the surface energy of the component metallic surface, we find that it is generally below 36 Dyne/cm and not in the 100s at all. The contaminants on the surface have greatly reduced the surface energy from the pure state of the metal. Surface energy is measured using water break tests or Dyne inks shown in Fig 4.
Therefore, we need to be able to measure the surface energies of the metal substrate and the liquid we are endeavouring to apply and ensure that the energy of the former is the higher value. Fig 3 shows that the surface energy of most metals in their pure state is greater than 400 Dynes/cm.
FIg 4. Dyne ink test
Why are these contaminants there and what can be done about them?
Lubricants are a major culprit.
Lubricants that are useful for one process are detrimental to another and the difference in surface energy between industrial surfaces and pure metallic surfaces is very often attributable to the lubricating fluids used during the forming process of the component. These hydrophobic agents work on the surface and are designed to reduce the friction between tool surfaces and the workpiece to speed up the manufacturing process, which they do very well. However, their presence is not conducive to forming reproducible, strong adhesive bonds. Wetting cannot occur properly and the lubricant is often unevenly spread over the surface leading to high variability in bonding and decrease in bond strength in wet environments.
A recent study undertaken to quantify the influence of a lubricant on an adhesively bonded aluminium joint confirmed both aspects lead to high variability in bond strength, whereas a thoroughly cleaned surface demonstrated excellent stability in both dry, wet and corrosive environments.
As well as using wettability tests, fluorescence techniques can be used to detect lubricants. Many organic substances show a fluorescence when illuminated with UV light.
Particulate contaminants - dust, soil and salts
The presence of larger particulates can be measured using simple tape pull tests, as described in ISO 8502-3, for macro evaluation. To detect finer particles and lower deposition levels, washing and filtration methods can be employed, as described in ISO 16232 Road vehicles- Cleanliness of components of fluid circuits. This method can also be used for qualitative determination of the composition of the contaminant.
The tape pull test is a useful guide to determine the level of particulates on the surface of incoming parts and a suitable cleaning process can be set up to effectively remove the surface contamination prior to subsequent chemical processes. Heavily soiled samples may require an additional pre-cleaning step before entering the automated process tanks. Not only will this ensure the effectiveness of the cleaning stage it also prevents contamination of the cleaning tank and helps to extend the life of the bath.
Cleaning Process
Removing particulates and oils
The main mechanisms for contamination removal from a surface in a typical alkaline, aqueous cleaner are shown in Fig 5. Most of the particulates and oils are held in suspension by an emulsification process. This consumes the emulsifier in the bath and eventually the efficiency of the tank decreases as more components pass through the tank. Although a scheduled tank dump and clean routine is commonly used in the industry to control the process this is not the most efficient method. The number of parts and the level of soil on the parts can vary significantly on a daily basis. At Powdertech Surface Science we take the view that it is as important to monitor the cleaning efficiency of the cleaner as it is the cleanliness of the parts exiting the process, to maintain a consistent output quality. Powdertech Surface Science use UV fluorescence alongside other monitoring methods to maintain efficiency of the cleaner tank and implement corrective action before the cleanliness of the parts begins to decline. This pre-emptive action helps to maintain a consistent part quality.
FIg. 5 Aqueous cleaning mechanisms
Passivation
Having a high surface energy to permit the adhesive to adequately wet the metal surface is the first step in achieving a strong bond. In addition, the metallic surface and the adhesive must have the right functionality to form a good bond. As described in table 1 weak boundary layers are not conducive to forming strong, stable bonds. In aluminium, weak boundary layers can be caused by both microstructural differences and/or oxides on the surface. These layers form part of the substrate and a subsequent chemical etching process is required to remove these layers to make them chemically clean, please see our Passivation of Aluminium White Paper for further details.
The robust and tested methods we employ at Powdertech determine the level of cleanliness of surfaces at the outset so that they can be cleaned and brought back into the state prior to powder coating and adhesive bonding operations. The coatings and the bonds formed with adhesives will fulfil the design life of the component.
Removing contaminants from metal prior to powder coating
Please contact us if you’d like to discuss our metal pre-treatment processes.
About the Author
Dr Nick Welton
Technical Consultant
Powdertech Surface Science
October 2022
Nick is a PhD polymer scientist with 24 years industrial experience in the field of polymers and coatings. Nick’s expertise includes powder coatings, material characterisation, specification testing, corrosion, weathering and failure analysis.